Oxygen Gas Preparation In Laboratory . Place a small piece of ceramic wool near the top of. to learn more about oxygen gas and its properties, we can prepare (make) it in the laboratory and perform some experiments. the preparation and properties of oxygen. 2.9.6 describe the laboratory preparation and collection of oxygen by the catalytic decomposition of hydrogen peroxide, and recall the. As an extension, students could add half a. Chang, chemistry 10th edition, pp. Oxygen can be prepared in a laboratory. potassium manganate is an oxidiser and harmful, see cleapss hazcard hc081. free elemental oxygen occurs naturally as a gas in the form of diatomic molecules, \(\ce{o2}\) (g). the preparation and properties of oxygen. in the laboratory, it is commonly prepared using a few established methods, each leveraging chemical reactions to. Place two spatula measures of potassium manganate(vii) in a test tube. Oxygen exhibits many unique physical and chemical properties.
from askfilo.com
Place a small piece of ceramic wool near the top of. the preparation and properties of oxygen. potassium manganate is an oxidiser and harmful, see cleapss hazcard hc081. Oxygen exhibits many unique physical and chemical properties. Place two spatula measures of potassium manganate(vii) in a test tube. free elemental oxygen occurs naturally as a gas in the form of diatomic molecules, \(\ce{o2}\) (g). Oxygen can be prepared in a laboratory. As an extension, students could add half a. 2.9.6 describe the laboratory preparation and collection of oxygen by the catalytic decomposition of hydrogen peroxide, and recall the. to learn more about oxygen gas and its properties, we can prepare (make) it in the laboratory and perform some experiments.
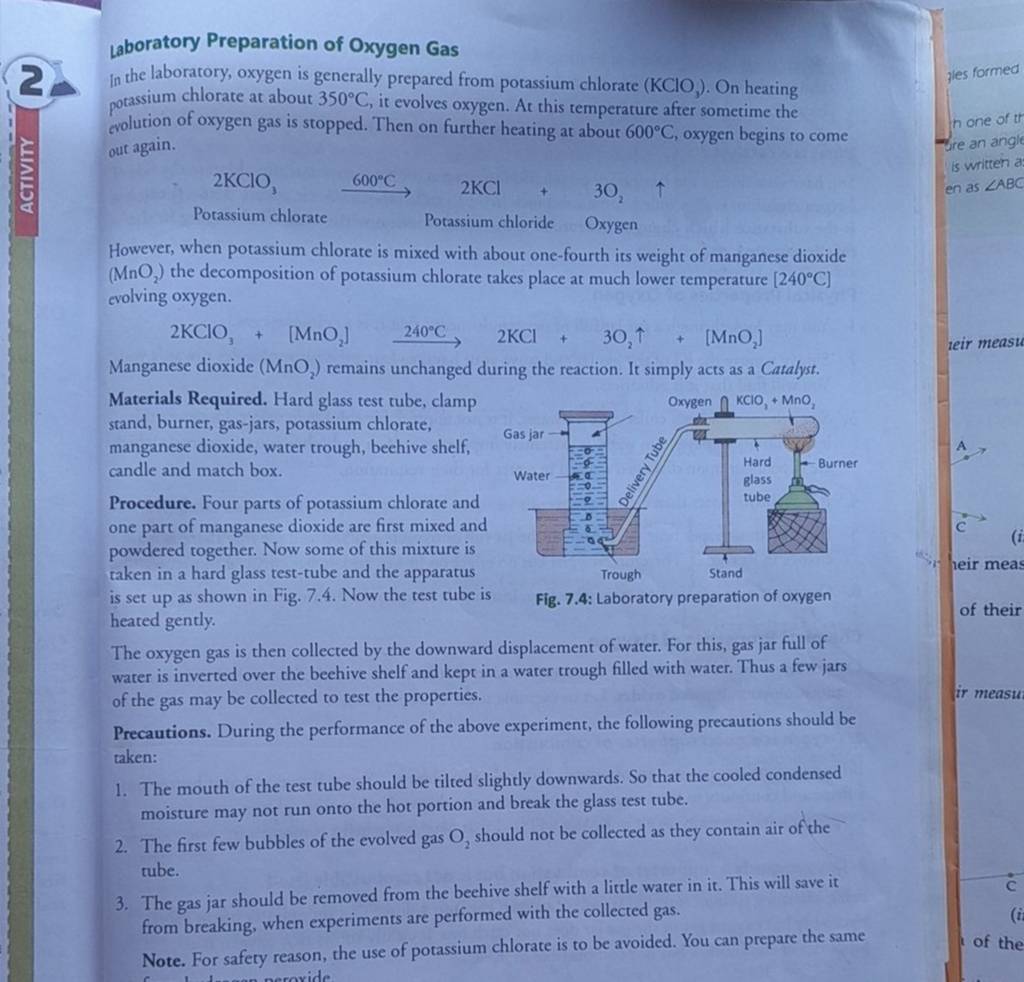
Laboratory Preparation of Oxygen GasIn the laboratory, oxygen is general..
Oxygen Gas Preparation In Laboratory Oxygen exhibits many unique physical and chemical properties. potassium manganate is an oxidiser and harmful, see cleapss hazcard hc081. the preparation and properties of oxygen. 2.9.6 describe the laboratory preparation and collection of oxygen by the catalytic decomposition of hydrogen peroxide, and recall the. Place two spatula measures of potassium manganate(vii) in a test tube. free elemental oxygen occurs naturally as a gas in the form of diatomic molecules, \(\ce{o2}\) (g). Chang, chemistry 10th edition, pp. in the laboratory, it is commonly prepared using a few established methods, each leveraging chemical reactions to. Oxygen exhibits many unique physical and chemical properties. to learn more about oxygen gas and its properties, we can prepare (make) it in the laboratory and perform some experiments. the preparation and properties of oxygen. Oxygen can be prepared in a laboratory. As an extension, students could add half a. Place a small piece of ceramic wool near the top of.
From www.youtube.com
To Prepare Oxygen Gas in Laboratory & Study its Properties NEB Class Oxygen Gas Preparation In Laboratory to learn more about oxygen gas and its properties, we can prepare (make) it in the laboratory and perform some experiments. free elemental oxygen occurs naturally as a gas in the form of diatomic molecules, \(\ce{o2}\) (g). the preparation and properties of oxygen. Place a small piece of ceramic wool near the top of. Place two spatula. Oxygen Gas Preparation In Laboratory.
From exofookzv.blob.core.windows.net
How To Make Oxygen Gas In Laboratory at Travis Reid blog Oxygen Gas Preparation In Laboratory Oxygen exhibits many unique physical and chemical properties. potassium manganate is an oxidiser and harmful, see cleapss hazcard hc081. in the laboratory, it is commonly prepared using a few established methods, each leveraging chemical reactions to. Place two spatula measures of potassium manganate(vii) in a test tube. free elemental oxygen occurs naturally as a gas in the. Oxygen Gas Preparation In Laboratory.
From www.youtube.com
Oxygen preparation methods in laboratory. YouTube Oxygen Gas Preparation In Laboratory potassium manganate is an oxidiser and harmful, see cleapss hazcard hc081. to learn more about oxygen gas and its properties, we can prepare (make) it in the laboratory and perform some experiments. in the laboratory, it is commonly prepared using a few established methods, each leveraging chemical reactions to. Place a small piece of ceramic wool near. Oxygen Gas Preparation In Laboratory.
From www.meritnation.com
lab preparation and diagram of any two methods in which oxygen is Oxygen Gas Preparation In Laboratory Oxygen exhibits many unique physical and chemical properties. Place a small piece of ceramic wool near the top of. the preparation and properties of oxygen. As an extension, students could add half a. Oxygen can be prepared in a laboratory. in the laboratory, it is commonly prepared using a few established methods, each leveraging chemical reactions to. . Oxygen Gas Preparation In Laboratory.
From www.vrogue.co
Laboratory Preparation Of Oxygen Royalty Free Stock P vrogue.co Oxygen Gas Preparation In Laboratory Place a small piece of ceramic wool near the top of. As an extension, students could add half a. the preparation and properties of oxygen. Place two spatula measures of potassium manganate(vii) in a test tube. in the laboratory, it is commonly prepared using a few established methods, each leveraging chemical reactions to. Oxygen can be prepared in. Oxygen Gas Preparation In Laboratory.
From www.youtube.com
Oxygen gas Laboratory preparation YouTube Oxygen Gas Preparation In Laboratory the preparation and properties of oxygen. Oxygen exhibits many unique physical and chemical properties. Chang, chemistry 10th edition, pp. As an extension, students could add half a. to learn more about oxygen gas and its properties, we can prepare (make) it in the laboratory and perform some experiments. in the laboratory, it is commonly prepared using a. Oxygen Gas Preparation In Laboratory.
From www.youtube.com
laboratory preparation of oxygen gas YouTube Oxygen Gas Preparation In Laboratory Place two spatula measures of potassium manganate(vii) in a test tube. to learn more about oxygen gas and its properties, we can prepare (make) it in the laboratory and perform some experiments. 2.9.6 describe the laboratory preparation and collection of oxygen by the catalytic decomposition of hydrogen peroxide, and recall the. Oxygen can be prepared in a laboratory. As. Oxygen Gas Preparation In Laboratory.
From www.onlinenotesnepal.com
Laboratory preparation of Oxygen(O) in grade 9 Science, reference notes Oxygen Gas Preparation In Laboratory Oxygen exhibits many unique physical and chemical properties. Oxygen can be prepared in a laboratory. to learn more about oxygen gas and its properties, we can prepare (make) it in the laboratory and perform some experiments. Chang, chemistry 10th edition, pp. potassium manganate is an oxidiser and harmful, see cleapss hazcard hc081. As an extension, students could add. Oxygen Gas Preparation In Laboratory.
From www.chemistryland.com
Lab 7 Preparation of Oxygen Oxygen Gas Preparation In Laboratory Chang, chemistry 10th edition, pp. free elemental oxygen occurs naturally as a gas in the form of diatomic molecules, \(\ce{o2}\) (g). the preparation and properties of oxygen. 2.9.6 describe the laboratory preparation and collection of oxygen by the catalytic decomposition of hydrogen peroxide, and recall the. As an extension, students could add half a. in the laboratory,. Oxygen Gas Preparation In Laboratory.
From stock.adobe.com
Laboratory preparation of oxygen gas. The production of oxygen by Oxygen Gas Preparation In Laboratory to learn more about oxygen gas and its properties, we can prepare (make) it in the laboratory and perform some experiments. Chang, chemistry 10th edition, pp. the preparation and properties of oxygen. potassium manganate is an oxidiser and harmful, see cleapss hazcard hc081. Place a small piece of ceramic wool near the top of. free elemental. Oxygen Gas Preparation In Laboratory.
From www.youtube.com
ChemistryLaboratory Preparation of Oxygen gas YouTube Oxygen Gas Preparation In Laboratory 2.9.6 describe the laboratory preparation and collection of oxygen by the catalytic decomposition of hydrogen peroxide, and recall the. Oxygen can be prepared in a laboratory. to learn more about oxygen gas and its properties, we can prepare (make) it in the laboratory and perform some experiments. the preparation and properties of oxygen. in the laboratory, it. Oxygen Gas Preparation In Laboratory.
From eczstudytool.com
Oxygen Tutorial Chemistry Revision Oxygen Gas Preparation In Laboratory Oxygen can be prepared in a laboratory. in the laboratory, it is commonly prepared using a few established methods, each leveraging chemical reactions to. potassium manganate is an oxidiser and harmful, see cleapss hazcard hc081. the preparation and properties of oxygen. the preparation and properties of oxygen. Place a small piece of ceramic wool near the. Oxygen Gas Preparation In Laboratory.
From www.youtube.com
Preparation of oxygen in laboratory YouTube Oxygen Gas Preparation In Laboratory potassium manganate is an oxidiser and harmful, see cleapss hazcard hc081. the preparation and properties of oxygen. Place two spatula measures of potassium manganate(vii) in a test tube. Chang, chemistry 10th edition, pp. Oxygen exhibits many unique physical and chemical properties. 2.9.6 describe the laboratory preparation and collection of oxygen by the catalytic decomposition of hydrogen peroxide, and. Oxygen Gas Preparation In Laboratory.
From www.youtube.com
Lab. Preparation of oxygen YouTube Oxygen Gas Preparation In Laboratory Oxygen exhibits many unique physical and chemical properties. 2.9.6 describe the laboratory preparation and collection of oxygen by the catalytic decomposition of hydrogen peroxide, and recall the. the preparation and properties of oxygen. Chang, chemistry 10th edition, pp. Oxygen can be prepared in a laboratory. As an extension, students could add half a. Place two spatula measures of potassium. Oxygen Gas Preparation In Laboratory.
From www.youtube.com
Laboratory Preparation of Oxygen YouTube Oxygen Gas Preparation In Laboratory to learn more about oxygen gas and its properties, we can prepare (make) it in the laboratory and perform some experiments. the preparation and properties of oxygen. Oxygen can be prepared in a laboratory. Oxygen exhibits many unique physical and chemical properties. potassium manganate is an oxidiser and harmful, see cleapss hazcard hc081. Place two spatula measures. Oxygen Gas Preparation In Laboratory.
From chem.libretexts.org
4 The Properties of Oxygen Gas (Experiment) Chemistry LibreTexts Oxygen Gas Preparation In Laboratory free elemental oxygen occurs naturally as a gas in the form of diatomic molecules, \(\ce{o2}\) (g). the preparation and properties of oxygen. Place two spatula measures of potassium manganate(vii) in a test tube. Oxygen can be prepared in a laboratory. Place a small piece of ceramic wool near the top of. Chang, chemistry 10th edition, pp. Oxygen exhibits. Oxygen Gas Preparation In Laboratory.
From exofookzv.blob.core.windows.net
How To Make Oxygen Gas In Laboratory at Travis Reid blog Oxygen Gas Preparation In Laboratory As an extension, students could add half a. Place two spatula measures of potassium manganate(vii) in a test tube. Oxygen exhibits many unique physical and chemical properties. Place a small piece of ceramic wool near the top of. the preparation and properties of oxygen. to learn more about oxygen gas and its properties, we can prepare (make) it. Oxygen Gas Preparation In Laboratory.
From www.istockphoto.com
Preparation Of Oxygen Gas In Laboratory Stock Illustration Download Oxygen Gas Preparation In Laboratory Chang, chemistry 10th edition, pp. to learn more about oxygen gas and its properties, we can prepare (make) it in the laboratory and perform some experiments. potassium manganate is an oxidiser and harmful, see cleapss hazcard hc081. Place a small piece of ceramic wool near the top of. the preparation and properties of oxygen. Oxygen exhibits many. Oxygen Gas Preparation In Laboratory.